The Netherlands publishes national guide to probiotics with antibiotics
The National Guide to clinically proven probiotics for use during antibiotic treatment was launched through a publication in the scientific journal BMC Gastroenterology.
 The invisible world
The invisible world
The National Guide to clinically proven probiotics for use during antibiotic treatment was launched through a publication in the scientific journal BMC Gastroenterology.
Read the publication in BMC Gastroenterology.
The guide, which covers eight products, contains an overview of clinically proven probiotics that can be used in conjunction with antibiotics to prevent antibiotic-associated diarrhoea (AAD). The guide serves as a tool for patients, consumers and health care practitioners such as GPs, dentists and pharmacists.
Antibiotics are a weapon in the fight against bacterial pathogens. However, antibiotic medicines often also affect our body’s own bacteria, which live in our intestines. These gut microbiota, or gut flora, are crucial to intestinal functioning and therefore essential to good health. Antibiotics can have a disruptive effect on gut flora and trigger a variety of adverse symptoms. One of the most common is antibiotic-associated diarrhoea (AAD), which occurs in one in four adults treated with antibiotics. The main culprits are broad-spectrum antibiotics such as amoxicillin (with enzyme inhibitor). Dispensed 1,2 million times a year, this is the most prescribed antibiotic in the Netherlands.
ARTIS-Micropia gave the go-ahead for the development of the national guide to clinically proven probiotics for use during antibiotic treatment at the ‘Know Yourself’ symposium held in late 2016. The guide published today contains a practical overview of probiotics that can be used in conjunction with antibiotics to prevent antibiotic-associated diarrhoea and recurring infections. Defined as ‘living micro-organisms which when administered in sufficient quantities will bring a health benefit to the host’, probiotics protect gut flora from the disruptive effects of antibiotic treatment, promote recovery and reduce the risk of recurring infections. The use of particular probiotics for a number of specific medical conditions is already broadly supported, with the European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and the World Gastroenterology Organisation publishing similar conclusions on the use of probiotics in conjunction with antibiotics for the prevention of AAD.
The guide was compiled using a general methodology consisting of three sequential steps. The first step (i) was the evaluation of relevant clinical studies on effective probiotics (specifically for AAD). This took the form of a systematic review that included only those studies that met the most rigorous methodological requirements, namely randomised, double-blind and placebo-controlled, with a clear definition of AAD and in which the probiotic was administered over a period that was at least equal to the period of antibiotic treatment. Next, (ii) a list of available probiotic products was compiled, on the basis of which (iii) a recommendation was drawn up of probiotic products that meet the criteria of effective product composition.
On the basis of these criteria, only 32 of the 128 studies identified in total were selected. The results of these studies were aggregated for each specific dairy product and dietary supplement currently marketed in the Netherlands. These products were then classified according to their proven effect as demonstrated in a minimum of one to three independent clinical studies. Products found to have an effect in at least three of the included studies were awarded three stars, those with effects in two studies were awarded two stars, and those with an effect in one study were awarded only one star. This led to a total of seven single-strain and multi-strain formulae demonstrated to be effective in groups treated with probiotics. The Lactobacillus rhamnosus GG (LGG) strain was shown to reduce the risk of antibiotic-associated diarrhoea the most, with a minimal daily dose of 2×10^9 (two trillion) colony-forming units (CFU) reducing the chances of AAD by a factor of three in at least three of the included studies. The table shows the formulae used in individual products and how these products scored on our criteria.
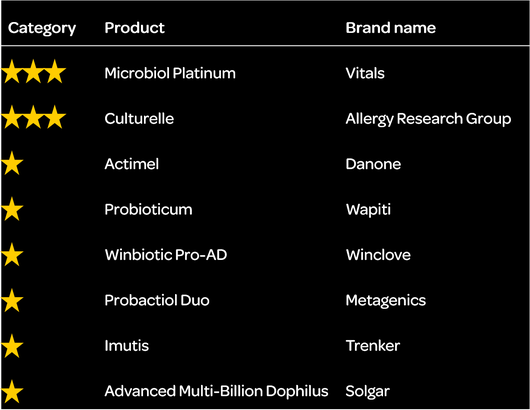
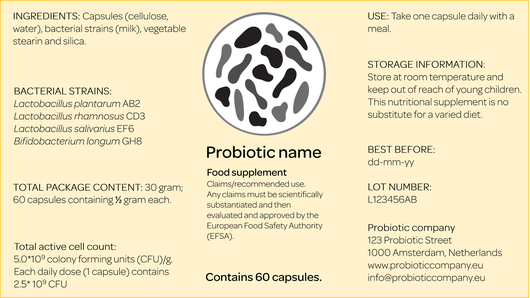
Products that did not specify probiotic strains and the number of CFUs on the label were excluded. Applying this criterion, only eight of the more than 140 probiotic products identified in the Dutch market could be recommended on the basis of the aforementioned studies (see table). However, this does not mean that the products not included are not effective. To address this deficiency, we are calling for the introduction of a standardised EU label on all probiotic products, specifying the probiotic strains and number of CFUs. Such labels are already in use in the United States, where producers are required to specify ingredients and hence the potency of the product. Taking the US label developed by the International Scientific Association for Probiotics and Prebiotics (ISAPP) as a model, we advocate a comparable label in the EU.
Though there is no scientific evidence available yet on the best treatment regime, the clinical studies included in the guide indicate that a good rule of thumb is to take probiotics over the whole period of antibiotic treatment (two hours after intake) and for another one to two weeks thereafter. The tool therefore now enables medical specialists, doctors and other healthcare practitioners to recommend specific probiotic products that are scientifically proven to prevent AAD. The national guide will be subject to annual evaluation and updates if and as new research that meets the inclusion criteria becomes available.
Read the publication.